One of the most common reasons stated by consumers to not buy electric vehicles is their range. On that front, things have improved over the last few years with EVs offering better range and faster charging. Still the perception remains that 300 to 400 miles is just not far enough, even though the vast majority of road trips are shorter than 10 miles. What would be the ideal figure for an EV range, 1,000 miles? If so, a new development may see that figure become reality.
A team of researchers from the Illinois Institute of Technology (IIT) and U.S. Department of Energy’s (DOE) Argonne National Laboratory have developed a lithium-air battery that could make it possible to travel over 1,000 miles on a single charge. The energy density of the new battery may also make it suitable for other new applications, such as powering aircraft.
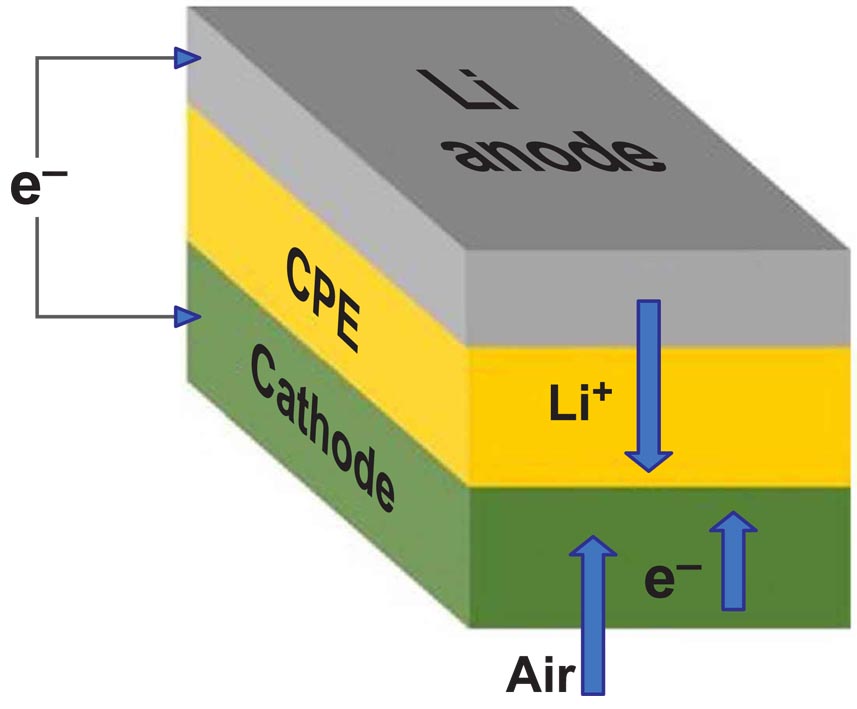 |
| Schematic shows lithium-air battery cell consisting of lithium metal anode, air-based cathode, and solid ceramic polymer electrolyte (CPE). On discharge and charge, lithium ions (Li+) go from anode to cathode, then back. |
The new battery uses lithium-air chemistry, but is different to other lithium-air battery designs in that it uses a solid electrolyte. The research team claims that the new battery chemistry has the potential to boost the energy density to four times more than current Li-ion designs. The solid electrolyte also brings another advantage in that it makes the battery much safer, by not being a fire hazard by spontaneously combusting. Liquid electrolytes also tend to be hazardous, and a solid electrolyte can’t leak.
In today’s lithium-air battery designs, the lithium in the lithium metal anode moves through a liquid electrolyte to combine with oxygen during the discharge, yielding lithium peroxide (Li2O2) or superoxide (LiO2) at the cathode. The lithium peroxide or superoxide is then broken back down into its lithium and oxygen components during charging. This allows the battery to store and release energy as it is needed. The newly developed solid electrolyte is composed of a ceramic polymer material made from relatively inexpensive elements in nanoparticle form. This new solid electrolyte enables chemical reactions that produce lithium oxide (Li2O) on discharge.
The new battery design is first lithium-air battery that has achieved a four-electron reaction at room temperature. It also takes the oxygen that is required from the air in the surrounding environment. This capability avoids the need for oxygen tanks to operate, which was a problem for earlier designs. The team used many different techniques to whether the four-electron reaction was occuring. One of these was transmission electron microscopy (TEM) of the discharge products on the cathode surface, which was carried out at Argonne’s Center for Nanoscale Materials.
“The chemical reaction for lithium superoxide or peroxide only involves one or two electrons stored per oxygen molecule, whereas that for lithium oxide involves four electrons,” said Argonne chemist Rachid Amine. More electrons stored means higher energy density.
One of the problems with lithium-air cells in the past is that they suffered from very short cycle lives. The research team who developed the new design built and operated a test cell, which showed that the battery worked for 1000 cycles, demonstrating stability over repeated charging and discharging.
